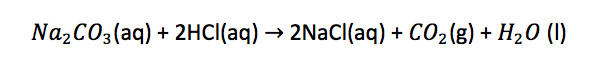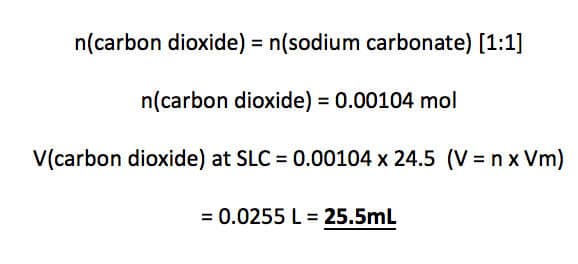Understanding Stoichiometry - VCE Chemistry
Stoichiometry is a term used to describe the calculation based on the chemical equations. It was a concept that was introduced to you in Unit 2 Chemistry and is a concept that is still very relevant in Year 12 Chemistry.
Many students find the concept of stoichiometry very difficult (I did as well!), however, once you get the hang of it you will find it very easy.
The key to stoichiometry is to ensure that you have the correct balanced chemical equation, without this your calculations will be incorrect. A chemical equation shows us the relative amounts of molecules (moles) participating in a chemical reaction.
When you come across a stoichiometric question, always ensure that you convert any numerical value you got into moles if possible. This is because, during stoichiometric calculations, you will be working in mole ratios. (It is much easier this way)
Excess and Limiting reagents:
Once you understand what is going with excess and limiting reagents you should be set to solving any type of stoichiometric question.
Sometimes in a question, you may be given the amounts of more than one reactant. Before you can calculate how much product will be formed, you need to work out which reactant is in excess and which reactant will be limiting (used up). The mole ratios from the chemical equation (either given or derived by you), is used to determine that.
It is best to explain this using an example.
Example (Adapted from Jacaranda Study On Chemistry 2 (2nd edition) p.21 Sample problem 1.8):
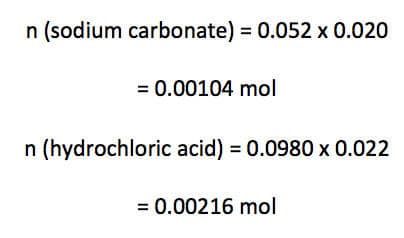
Calculate the volume of carbon dioxide gas evolved (at SLC) when 20.0 mL of 0.052M sodium carbonate solution is mixed with 22.0 mL of 0.0980M hydrochloric acid.
Solution:
Derive a chemical equation:
Convert numerical amounts into moles:
From the equation, 1 mole of sodium carbonate requires 2 moles of hydrochloric acid. (1:2 ratio)
Therefore, 0.00104 mol of sodium carbonate requires 0.00208 mol (0.00104 x 2) of hydrochloric to react with. We have 0.00216 mol of hydrochloric acid, therefore, hydrochloric acid is in excess and sodium carbonate is the limiting reagent.
*Once you have found which reagent is in excess and which is limiting, you would use the reagent that is limiting in the rest of your calculations because your reaction is ‘limited’ by the amount of that particular reagent. ➔ The reaction will stop if that reagent is used up.
For more practice, go back to your Year 11 Chemistry textbook and do some of the stoichiometric questions. Similarly, the first few chapters of your Year 12 Chemistry textbook should have some stoichiometric questions as well.
Have fun!
About Learnmate
Learnmate is a trusted Australian community platform that connects students who want 1:1 or small group study support, with tutors who are looking to share their knowledge and earn an income. From primary school to high school subjects — from science and maths to niche subjects like visual communication — Learnmate can help you improve academic performance or boost confidence, at your pace with the tutor that you choose.
We pride ourselves in offering a reliable and positive experience for both our students and tutors. Every tutor that joins the platform is vetted to meet a level of academic excellence, teaching qualification or relevant experience. All tutors are provided the opportunity to complete professional training.
Students and parents can easily find and screen for tutors based on their location, their subject results or skill level, and whether they provide in-person or online sessions. Learnmate is proud to provide tutors in Melbourne, Sydney, Geelong, Brisbane, Hobart, Canberra, Perth & Adelaide, and other locations.